Digital Braille and Tactile Labels
Home / Full Product Range / Digital Labels / Digital Braille and Tactile Labels
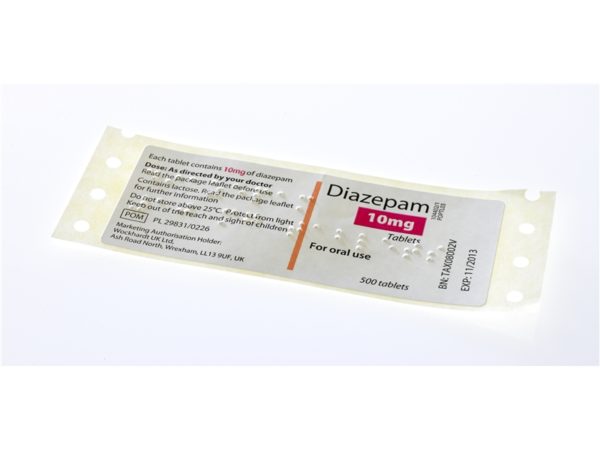
An exciting new innovation from Labelservice is the ability to offer inline digitally printed braille and tactile labels. Previously only available on large production runs and with prohibitive tooling and plate costs, we can now supply braille and tactile warning labels with all of the advantages that digital technology brings, such as multi sort printing, unique and variable imagery and full press proofing.
Please contact us for further information and samples.
Braille Tactile Labels
Braille tactile labels are specialised labels embossed with braille characters to provide essential information about a product to visually impaired individuals. These labels are designed to be easily readable by touch, ensuring that the information is accessible to all, regardless of visual ability. Braille tactile labels are particularly crucial for products like medicines and chemicals, where conveying accurate information is paramount for safe usage. As a professional label manufacturer, we ensure that our braille tactile labels and braille tactile stickers adhere to the highest standards of quality and accessibility.
Braille tactile labels are important for ensuring the safe use of medicines and chemical products by visually impaired individuals. They convey crucial safety information and dosage instructions, mitigating the risks of misuse. The inclusion of braille on packaging is a legal requirement in many regions, including the UK, ensuring compliance with accessibility standards. Also, using braille tactile labels and braille tactile stickers reflects a commitment to inclusivity by the manufacturers, demonstrating a brand’s consideration for a diverse consumer base.
Braille tactile labels are created using specialised embossing or printing techniques that render the braille characters in a raised format on the label material. The process begins with the design and translation of text into braille, followed by the selection of a suitable material for the label. The braille characters are then embossed or printed onto the label using high-precision equipment to ensure accurate and readable braille translation.
Yes, braille tactile labels are designed to withstand harsh chemical environments. They are often manufactured from durable materials such as polyester or polyethylene, which are known for their chemical resistance and longevity. These materials ensure that the braille characters remain legible and the label retains its integrity even when exposed to harsh chemicals or extreme conditions.
Yes, braille tactile labels are a legal requirement for medicines in the UK. According to the European Union regulations, which the UK adhered to, it’s mandatory to have braille on the packaging of medicinal products to ensure they are accessible to visually impaired individuals.
For chemical based products, under the regulations set out called CLP, there isn’t a specific legal requirement in the UK for braille tactile labels to be used on chemical products similar to the requirement for medicinal products. However, manufacturers are encouraged to adhere to best practices in labelling to ensure safety and accessibility for all users, which can include the use of braille tactile labels and stickers.
Braille tactile labels for medicines typically include essential information such as:
Product Name: The name of the medicine.
Strength: The concentration or potency of the medicine.
Dosage Instructions: Guidance on how to administer the medicine.
For chemical products, braille tactile labels or braille tactile stickers may include:
Product Name: The name of the chemical or product.
Hazard Warnings: Warnings regarding the hazards associated with the chemical.
Safety Instructions: Instructions on safe handling and usage.
An exciting new innovation from Labelservice is the ability to offer inline digitally printed braille and tactile labels. Previously only available on large production runs and with prohibitive tooling and plate costs, we can now supply braille and tactile warning labels with all of the advantages that digital technology brings, such as multi sort printing, unique and variable imagery and full press proofing.
Please contact us for further information and samples.
Braille Tactile Labels
Braille tactile labels are specialised labels embossed with braille characters to provide essential information about a product to visually impaired individuals. These labels are designed to be easily readable by touch, ensuring that the information is accessible to all, regardless of visual ability. Braille tactile labels are particularly crucial for products like medicines and chemicals, where conveying accurate information is paramount for safe usage. As a professional label manufacturer, we ensure that our braille tactile labels and braille tactile stickers adhere to the highest standards of quality and accessibility.
Braille tactile labels are important for ensuring the safe use of medicines and chemical products by visually impaired individuals. They convey crucial safety information and dosage instructions, mitigating the risks of misuse. The inclusion of braille on packaging is a legal requirement in many regions, including the UK, ensuring compliance with accessibility standards. Also, using braille tactile labels and braille tactile stickers reflects a commitment to inclusivity by the manufacturers, demonstrating a brand’s consideration for a diverse consumer base.
Braille tactile labels are created using specialised embossing or printing techniques that render the braille characters in a raised format on the label material. The process begins with the design and translation of text into braille, followed by the selection of a suitable material for the label. The braille characters are then embossed or printed onto the label using high-precision equipment to ensure accurate and readable braille translation.
Yes, braille tactile labels are designed to withstand harsh chemical environments. They are often manufactured from durable materials such as polyester or polyethylene, which are known for their chemical resistance and longevity. These materials ensure that the braille characters remain legible and the label retains its integrity even when exposed to harsh chemicals or extreme conditions.
Yes, braille tactile labels are a legal requirement for medicines in the UK. According to the European Union regulations, which the UK adhered to, it’s mandatory to have braille on the packaging of medicinal products to ensure they are accessible to visually impaired individuals.
For chemical based products, under the regulations set out called CLP, there isn’t a specific legal requirement in the UK for braille tactile labels to be used on chemical products similar to the requirement for medicinal products. However, manufacturers are encouraged to adhere to best practices in labelling to ensure safety and accessibility for all users, which can include the use of braille tactile labels and stickers.
Braille tactile labels for medicines typically include essential information such as:
Product Name: The name of the medicine.
Strength: The concentration or potency of the medicine.
Dosage Instructions: Guidance on how to administer the medicine.
For chemical products, braille tactile labels or braille tactile stickers may include:
Product Name: The name of the chemical or product.
Hazard Warnings: Warnings regarding the hazards associated with the chemical.
Safety Instructions: Instructions on safe handling and usage.